No products in the cart.
Easy to buy
Variety mode of payment
Easy to buy
Variety mode of payment
Free shipping
For orders from 3,000,000 VND
Support 24/7
Even outside working hour
Contact Ho Chi Minh City
Tel: (028).3977.8269 / 0906.988.447
Email: sales@lidinco.com
Contact Bac Ninh, Ha Noi City
Tel: (0222).730.0180
Email: bn@lidinco.com
Built-in timer – Display of time remaining before a measurement is taken. Ensures that all readings are taken at the appropriate reaction intervals for the test being performed.
Zero key – A simple press of the zero key on the face of the meter will account for the color and imperfections in the oil sample before reagent addition.
Auto shut-off – Automatic shut-off after 15 minutes of non-use when the meter is in measurement mode. Prevents wastage of batteries in the event the meter is accidentally left on.
Battery status indicator – Indicates the amount of battery life left.
Error messages – Messages on display alerting to problems including no light, inverted sample, and out of range.
Units of measure – Appropriate unit of measure is displayed along with reading.
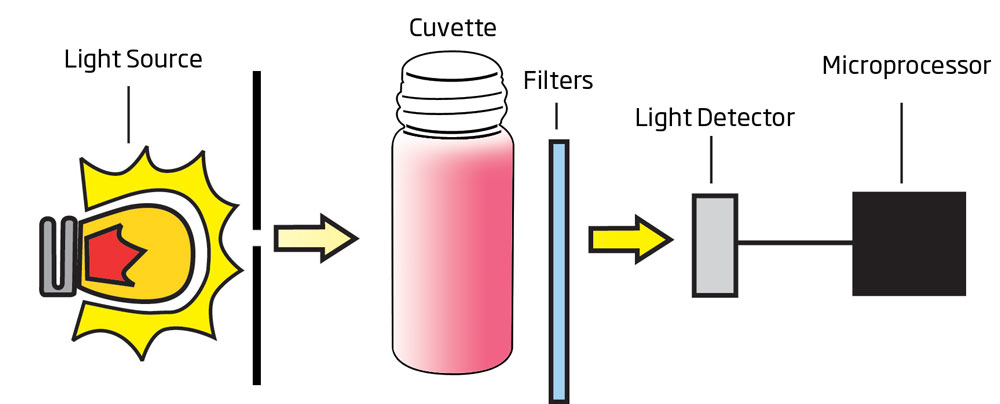
Over time, edible oils may degrade and spoil. The primary cause of edible oil degradation is oxidation; as oil oxidation occurs, flavors and odors can change, resulting in a product that is undesirable to consumers. The unsaturated fatty acids found in oils react with oxygen, creating peroxide as an unwanted byproduct. This oxidation reaction is more likely to occur under certain conditions, including exposure to light, the presence of metal ions, the introduction of oxygen, or when storage temperatures are not maintained. In order to determine oil quality and the onset of oxidation, peroxide value is determined. Peroxide value is defined as the amount of peroxide oxygen per kilogram of oil, which is reported in units of milliequivalents or meq. A lower peroxide value indicates higher quality edible oil.
The HI83730 uses an adaptation of the EC 2568/91 method and following amendments to measure peroxide values of less than 25.0 meq O2/kg. When the reagent is added to a sample containing peroxides, the sample will turn a yellow hue; the greater the concentration, the deeper the color. The associated color change is then colorimetrically analyzed according to the Beer-Lambert Law. This principle states that light is absorbed by a complementary color, and the emitted radiation is dependent upon concentration. For determination of peroxide value, a narrow band interference filter at 466 nm (blue) allows only blue light to be detected by the silicon photodetector and omits all other visible light emitted from the tungsten lamp. As the change in color of the reacted sample increases, absorbance of the specific wavelength of light also increases, while transmittance decreases.
| Peroxide in Oil Range | 0.0 to 25.0 meq O₂/kg |
|---|---|
| Peroxide in Oil Resolution | 0.5 meq O₂/kg |
| Peroxide in Oil Accuracy | ±0.5 meq O₂/kg |
| Peroxide in Oil Method | adaptation of EC 2568/91 method and following amendments |
| Photometer/Colorimeter Light Source | tungsten lamp with narrow band interference filter @ 466 nm |
| Photometer/Colorimeter Light Detector | silicon photocell |
| Display | Liquid Crystal Display (LCD) |
| Power Supply | 4 (1.5V) AA batteries or 12 VDC adapter |
| Battery Type/Life | 1.5V AA batteries |
| Automatic Shut-Off | after 15 minutes of non use |
| Environment | 0 to 50°C (32 to 122°F); RH max 95% non-condensing |
| Weight | 512 g (18 oz) |
| Dimensions | 224 x 87 x 77 mm (8.8 x 3.4 x 3”) |
| Ordering Information | HI83730 is supplied with reagents for 10 tests, 4 (1mL) graduated syringes, scissors, cuvette cleaning cloth, 4 (1.5V) AA batteries, AC adapter, instruction manual, and a rigid carrying case. |